An atom containing one proton is a hydrogen atom (H). An atom containing 6 protons is a carbon atom. And an atom containing 8 protons is an oxygen atom. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. Dell modems driver download for windows 10. The atom is electrically neutral when the atom is not negatively charge or positively charged. In other words, it doesn't have more electrons than protons and more protons than electrons.
What determines if an atom is electrically charged or electrically neutral?
1 Answer

Explanation:
Electric charge is determined by subatomic particles called 'electrons' and 'protons'. Electrons have a -1 negative charge while protons have a +1 positive charge.
By looking at the periodic table, each element's atomic number is equal to the protons and electrons it has when it is electrically neutral. Neutrality is classified as a net 0 electric charge (ex. 2 protons and 2 electrons in neutral Helium create the electrical equation
Atoms are not always electrically neutral, we call these atoms 'ions'.
Atoms that have more electrons than protons are classified as 'anions' and atoms that have more protons than electrons are classified as 'cations'.
This is atomic charge summed up without going into too much detail, it's simply determined by electrons and protons in an atom.
Related questions
Atom Is Electrically Neutral Assertion And Reason
Click to see full answer.
Also, why is an atom electrically neutral quizlet?
An atom is electrically neutral because the number of negatively charged electrons outside the nucleus equals the number of positively charged protons inside the nucleus. You can only create an ion by adding or removing electrons from a neutral atom.
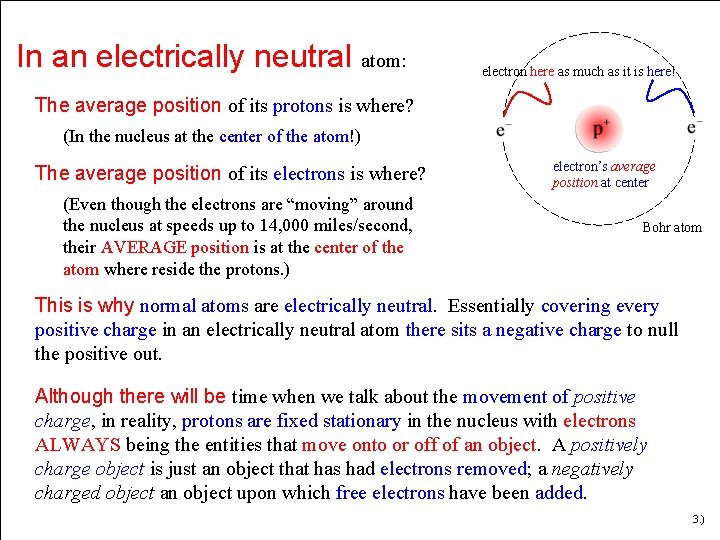
Subsequently, question is, is the nucleus of an atom electrically neutral? An atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles called electrons. The positive charges equal the negative charges, so the atom has no overall charge; it is electrically neutral. The nucleus of an atom contains protons and neutrons.
An Atom Is Electrically Neutral Because
Likewise, people ask, what elements are neutral atoms?
A neutral atom is an atom where the charges of the electrons and the protons balance. Luckily, one electron has the same charge (with opposite sign) as a proton. Example: Carbon has 6 protons. The neutral Carbon atom has 6 electrons.
Brew os. Emuzed driver. Why neutron is electrically neutral?
Neutrons are the particles in an atom that have a neutral charge. They aren't positive like protons. They aren't negative like electrons. So, if an atom has equal numbers of electrons and protons, the charges cancel each other out and the atom has a neutral charge.
